Energy dispersive X-ray fluorescence (EDXRF), commonly referred to as XRF, is a fast, nondestructive method to measure the elemental composition of a material. But how does XRF work?
Here, we will discuss the science behind XRF and explain how it works in handheld analyzers.
The Science Behind Energy Dispersive XRF
X-ray energy forms the basis for insightful measurement techniques. Energy dispersive X-ray fluorescence identifies metals and elements in an object by detecting their signature XRF emission energies.
Here’s a detailed explanation of the process:
Essentially, all elements have a fixed number of electrons arranged in atomic orbitals around their nuclei. When photons from the X-ray tube strike the object of interest with enough energy to expel the electrons out of the elements’ innermost orbitals, the atoms become unstable.
To regain stability, electrons from the outer orbitals move into the newly vacant spaces in the inner orbitals. As an electron transitions from an outer orbital to an inner orbital, it emits photon energy known as X-ray fluorescence. This release of energy is illustrated in the image below.
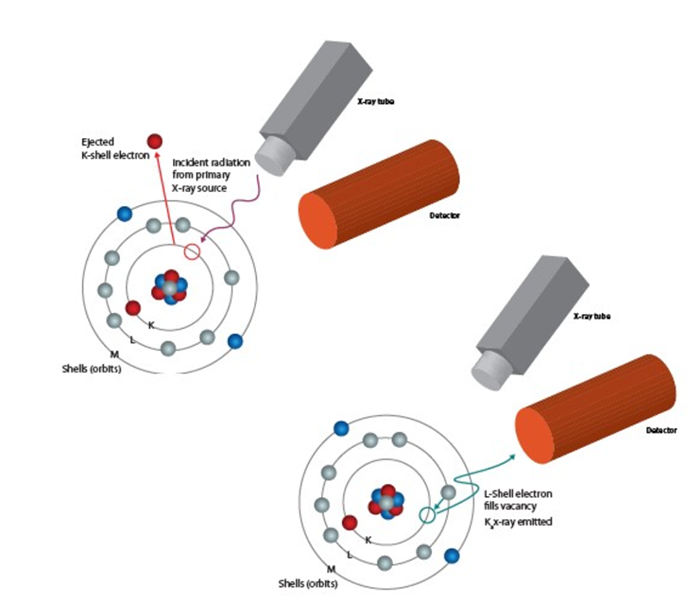
This energy is determined by the different energies between the initial and final orbitals of the individual’s electron transitions.
The amount of element present in an object is determined by the intensity of the signal detected at its signature energy. For example, if lead (Pb) is present in an object, an XRF signal will be detected at 10.55 and 12.61 keV, and its quantity can be determined by plotting the energy (E) vs. intensity (I).
How Handheld XRF Works
Now that you understand the science behind XRF, you might be wondering how this technology works in handheld XRF analyzers like our popular Vanta™ series.
The handheld XRF process can be broken down into four simple steps:
1. Emission
First, the analyzer emits X-rays.

2. Excitation
The X-rays hit the sample, causing it to fluoresce and send X-rays back to the analyzer.
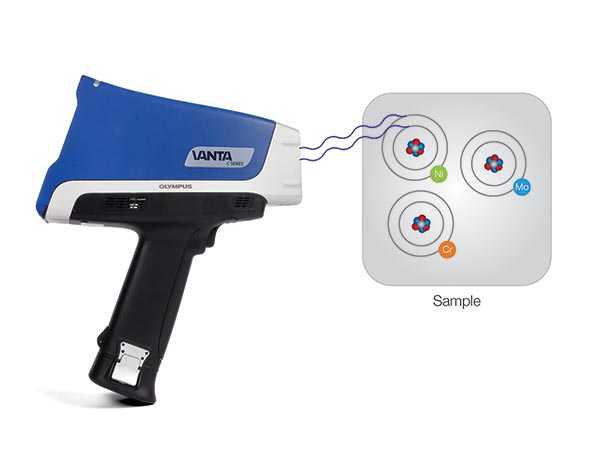
3. Measurement
The detector measures the energy spectrum. This tells you which elements are present and the amount of each element present. It’s important to note that Olympus XRF can’t be used to measure every element in the periodic table. In general, our analyzers can measure from phosphorus to plutonium (P–Pu) on the periodic table of elements.

4. Results
Olympus Axon Technology™ processes the energy spectrum and displays the sample’s elemental composition. For metals, we match the composition to a particular grade.

How Can These XRF Results Be Used?
XRF analyzers do complicated math so you can focus on getting fast, accurate results when and where you need it. You can use these fast elemental analysis and alloy identification results for a wide range of purposes. Common XRF applications include:
- Material verification
- Scrap recycling
- Mining and geochemistry
- Environmental assessments
- Education
- Regulatory and safety screening
- Precious metals analysis
To learn more about XRF technology, check out these X-Ray Fluorescence FAQs next.
Related Content
X-Ray Fluorescence (XRF) Frequently Asked Questions (FAQs)
Infographic: Vanta Handheld XRF Analyzers
Portable XRF: A Quickstart Guide for Best Practices

