Title 21 Part 11 of the Code of Federal Regulations (CFR) defines the criteria for electronic data integrity as set by the United States Food and Drug Administration (FDA). FDA-regulated industries, such as medical device manufacturers and pharmaceutical companies, must comply with 21 CFR Part 11 to ensure accurate electronic records and signatures.
Yet, complying with these requirements can be difficult and time-consuming. This blog will explore common challenges of meeting 21 CFR Part 11 compliance and discuss ways to ease them.
The Challenges of Meeting 21 CFR Part 11 Compliance
Compliance typically involves creating work instructions to meet each specific 21 CFR Part 11 regulation, developing validation protocols to ensure proper setup, operation, and performance of equipment and software, creating standard operating procedures (SOPs), and extensive training for personnel using the equipment and software.
Any tool that makes this process easier is helpful.
Many manufacturers in FDA-regulated industries use our OLYMPUS CIX100 technical cleanliness inspector. Built with an intuitive interface, efficient data acquisition, and rapid reporting options, the CIX100 system can help you quickly and easily evaluate the cleanliness of manufactured components to determine if they adhere to company and international standards.
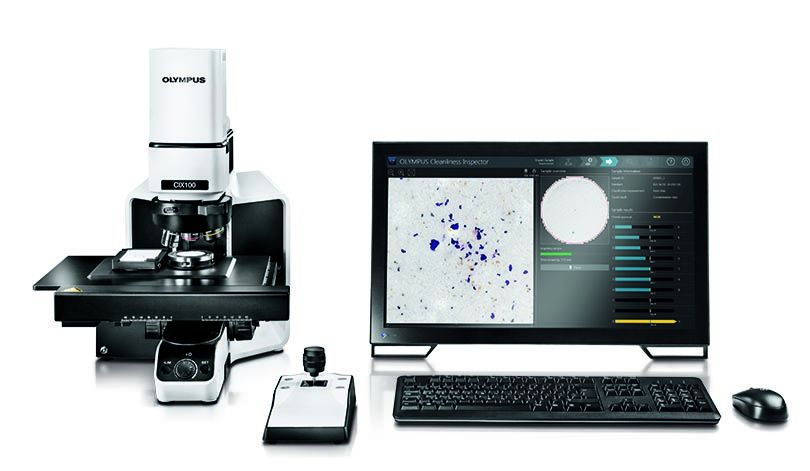
The OLYMPUS CIX100 cleanliness inspector.
To help you easily comply with these requirements, we created a 21 CFR Part 11 package for the CIX100 cleanliness inspector.
Simplifying 21 CFR Part 11 Compliance Using the CIX100 Cleanliness Inspector
Our CIX100 21 CFR Part 11 package demonstrates how the CIX100 system can achieve compliance with each requirement and provides documents for system setup and operation. When the package is implemented properly, the CIX100 system will comply with 21 CFR Part 11.
The package includes:
- Documents detailing how the CIX100 system with Adobe Sign meets specific 21 CFR Part 11 requirements
- CIX100 validation protocol for installation qualification/operational qualification (IQ/OQ)
- Standard operating procedure (SOP) for the CIX100 system in compliance with 21 CFR Part 11
- Work instructions for the setup and operation of the CIX100 system in compliance with 21 CFR Part 11
A Roadmap to 21 CFR Part 11 Compliance
With these detailed resources, the package clearly lays out what you need to know to meet the requirements of 21 CFR Part 11. This includes what solutions we provide, and any remaining user responsibilities. Here’s a quick overview:
Olympus solutions to 21 CFR Part 11 regulations include:
- Validating the system for accurate and reliable results through IQ/OQ
- Providing CIX100 training to microscope users
- Ensuring proper operation with a detailed SOP
- Restricting system access to authorized individuals through a Windows PC login and user rights management
- Using Adobe Sign to provide electronic signatures and track PDF reports of sample inspections
User responsibilities include:
- Provide training for Adobe Sign for electronic signatures and tracking of electronic data
- Create policies for using CIX100 software with Adobe Sign and for tracking access and revisions to previous sample inspection reports
- Maintain procedures for loss management and document retention
With this package, the CIX100 system can help you streamline cleanliness inspections and meet compliance with these important FDA standards for electronic records and signatures.
Related Content
An Introduction to Technical Cleanliness Inspection
5 Ways the OLYMPUS CIX100 Cleanliness Inspector Delivers Reproducible Results
Brochure: Explanation of Standards for Technical Cleanliness
Get In Touch

